Hypervalent Iodine Chemistry
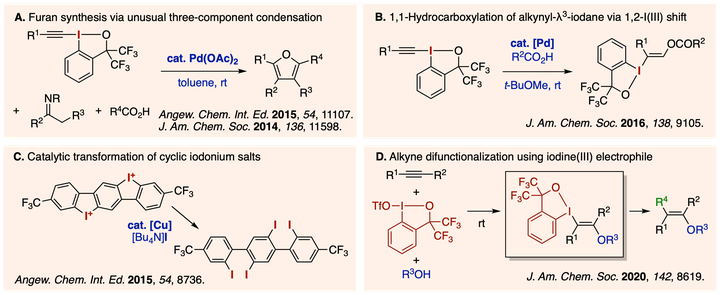
Hypervalent iodine compounds have received considerable interests from chemists as environmentally friendly oxidants as well as versatile group-transfer reagents for organic synthesis. Our foray into hypervalent iodine chemistry started with a serendipitous discovery of a palladium-catalyzed condensation reaction between imines and ethynylbenziodoxolones to form multisubstitituted furans, involving unusual reactivity pattern of the electrophilic alkynyl-transfer agents. Since then, we have been exploring novel reactivities of iodine(III) compounds, especially those featuring cyclic benziodoxole skeletons, as electrophilic and group-transfer reagents for efficient and selective organic synthesis.
Selected Publications
Ding, W.; Chai, J.; Wang, C.; Wu, J.; Yoshikai, N. Stereoselective Access to Highly Substituted Vinyl Ethers via trans-Difunctionalization of Alkynes with Alcohols and Iodine(III) Electrophile. J. Am. Chem. Soc. 2020, 142, 8619-8624.
Wu, J.; Deng, X.; Hirao, H.; Yoshikai, N. Pd-Catalyzed Conversion of Alkynyl-λ3-iodanes to Alkenyl-λ3-iodanes via Stereoselective 1,2-Iodine(III) Shift/1,1- Hydrocarboxylation. J. Am. Chem. Soc. 2016, 138, 9105-9108.
Lu, B.; Wu, J.; Yoshikai, N. Palladium-Catalyzed Condensation of N-Aryl Imines and Alkynylbenziodoxolones To Form Multisubstituted Furans. J. Am. Chem. Soc. 2014, 136, 11598-11601.