Stereoselective Access to Multisubstituted Vinyl Ethers via trans-Difunctionalization of Alkynes with Alcohols and Iodine(III) Electrophile
Abstract
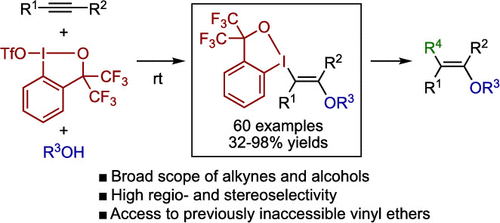
A method for the regio- and stereoselective synthesis of highly substituted vinyl ethers via trans-1,2- difunctionalization of alkynes with a cyclic λ3-iodane electrophile (benziodoxole triflate) and alcohols is reported. The reaction tolerates a variety of internal and terminal alkynes as well as various alcohols, affording β-λ3-iodanyl vinyl ethers in good yields with high regio- and stereoselectivities. The benziodoxole moiety of the products can be used as a versatile linchpin for the synthesis of structurally diverse vinyl ethers that are difficult to access by other means.
Type
Publication
Ding, W.; Chai, J.; Wang, C.; Wu, J.; Yoshikai, N. J. Am. Chem. Soc. 2020, 142, 8619-8624.