Research
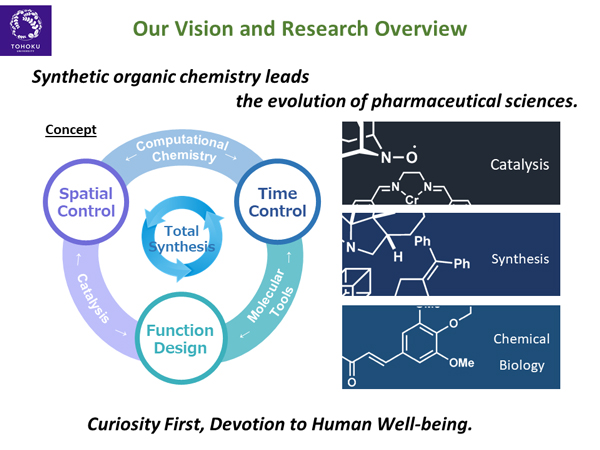
Based on a high degree of intellectual curiosity, the Iwabuchi Laboratory conducts research on the synthetic organic chemistry of fine chemicals to advance pharmaceutical sciences.
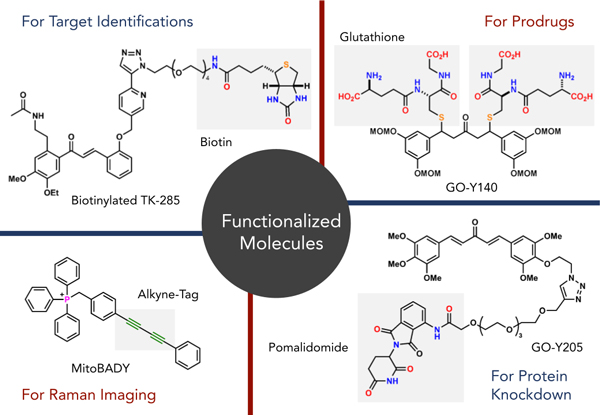
An important molecular function in pharmaceutical sciences is biological activity. Therefore, the Iwabuchi Laboratory investigates the synthesis of bioactive compounds (mainly natural products) as the main subject. We are also developing catalytic reactions that enable efficient synthesis of bioactive compounds and pharmaceuticals, and chemical biology research through the creation of molecular tools to clarify the mechanisms of activity of bioactive compounds and pharmaceuticals.
In catalytic reaction development, we are particularly interested in asymmetric catalysis. Precise control of the reaction space with original molecules is our important philosophy for this purpose. In the development of molecular tools, we aim to elucidate not only where and how compounds act, but also their changes over time (temporal control). To achieve these highly challenging spatial and temporal controls, we utilize not only experimental chemistry but also computational chemistry to efficiently carry out our research.
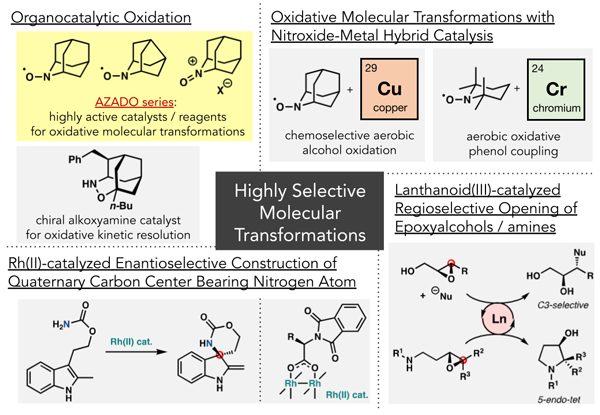
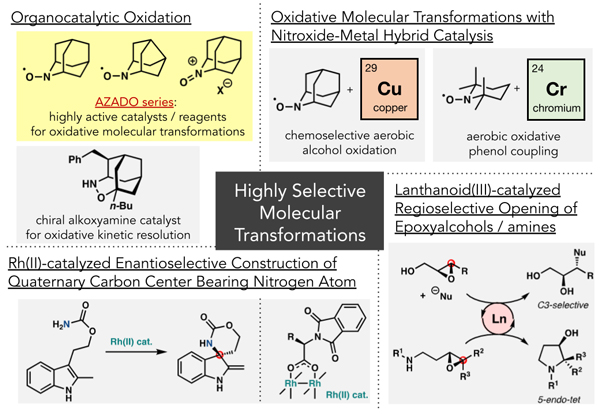
Development of Highly Selective Organic Synthesis Methodologies
- ・Organocatalytic oxidation reactions
- ・Oxidative molecular transformations using organocatalyst-metal hybrid catalysts
→Chemoselective aerobic alcohol oxidation using nitroxyl radical/copper catalysis
→Oxidative phenol coupling using nitroxyl radical/chromium catalysis - ・Asymmetric construction of nitrogen-containing tetrasubstituted carbon centers using chiral Rh(II) catalysis
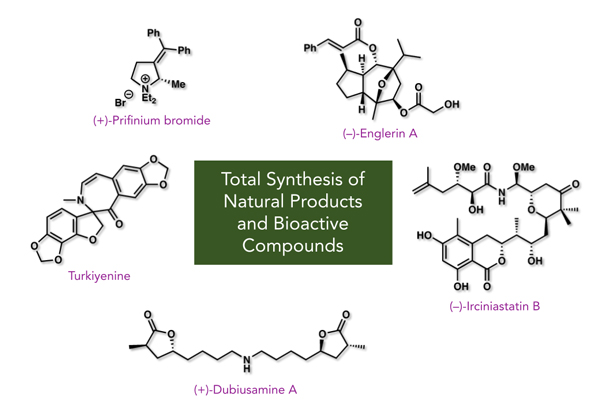
Stereocontrolled Synthesis of Bioactive Natural Products
- Synthesized natural products and pharmaceuticals (scince 2010)
- ・Sarinfacetamide A, B (Chemical Science 2025, 16, 15707)
- ・Cytotrienin A (Angew. Chem. Int. Ed. 2023, e202303140, )
- ・Rumphellclovane E (Org. Lett. 2022, 24, 7572)
- ・(+)-Nemonapride (Chem. Pharm. Bull. 2017, 65, 22-24)
- ・Heronamides A-C (Chem. Eur. J. 2016, 22, 8586-8595)
- ・FD-891 (J. Antibiot 2016, 69, 287-293)
- ・Turkiyenine (Proposed Structure) (Eur. J. Org. Chem 2016, , 270-273)
- ・Irciniastatins A (Psymberin) and B (J. Org. Chem 2015, 80, 12333-12350)
- ・(+)-Dubiusamine A (Org. Lett 2013, 15, 1788-1790)
- ・Sundiversifolide (formal synthesis) (Org. Lett 2011, 13, 3620-3623)